Review Article
Year: 2020 | Month: March | Volume: 7 | Issue: 3 | Pages: 435-447
Novel Coronavirus (COVID-19) in India: Current Scenario
Varsha Kachroo
Resident, Dept. of Medicine, Govt. Medical College, Jammu.
ABSTRACT
Covid-19, just few days back, was foreign to us and now spreading its routes well in India. Starting with one case and now with 800+ cases, the virus is trending right now in almost every part of the country. The irony is not much is known about this novel virus, hence mortality and morbidity across the globe is on a peak. The Ministry of Health and Family Welfare, Government of India and ICMR (Indian Council of Medical Research) has formulated guidelines, advisories for social distancing protocol, diagnosis, management, do’s and don'ts and other reliable material. The topic of Novel Corona virus is huge to cover it entirely in every aspect on a single page, and so is its impact across the world. But the basic things and protocols remain same everywhere. This review briefly covers the introduction, possible mode of transmission, definitions, some basic advices, diagnosis, treatment and management protocol being followed right now in India; however subjected to change in due course of time as is the number of cases and mortality. The health care personnel are doing their job perfectly and so is the Government, but what is important for everyone being a citizen of India is to maintain social distancing and follow advisories strictly from time to time so that we can make way for our own lives and lives of our dear ones.
Keywords: Covid-19, coronavirus, India
Published: 28th March 2020
INTRODUCTION
Definitions and Triage: [2]
Currently in India, following definitions are used to define and triage patient with infection.
SARI (Severe Acute Respiratory Infection): An ARI with history of fever or measured temperature ≥38 °C and cough; onset within the last ~10 days; and requiring hospitalization.
Current case definition: [2]
1. SARI in a person, with history of fever and cough requiring admission to hospital, with no other etiology that fully explains the clinical presentation (testing should be according to local guidance for management of community-acquired pneumonia. Examples of other etiologies include Streptococcus pneumoniae, Haemophilus influenza type B, Legionella pneumophila, other recognized primary bacterial pneumonias, influenza viruses, and Respiratory syncytial virus. Clinicians should also be alert to the possibility of atypical presentations in patients who are immunocompromised);
AND any of the following:
a) A history of international travel in 14 days prior to symptom onset; or
b) the disease occurs in a health care worker who has been working in an environment where patients with SARI are being cared for, without regard to place of residence or history of travel; or
c) the person develops an unusual or unexpected clinical course, especially sudden deterioration despite appropriate treatment, without regard to place of residence or history of travel, even if another etiology has been identified that fully explains the clinical presentation
2. A person with acute respiratory illness of any degree of severity who, within 14 days before onset of illness, had any of the following exposures:
a) Close physical contact (defined below) with a confirmed case of COVID - 19 infection, while that patient was symptomatic; or
b) A healthcare facility in a country where hospital-associated COVID - 19 infections have been reported;
CLOSE CONTACT DEFINITION: [2]
- Health care associated exposure, including providing direct care for COVID-19 patients, working with health care workers infected with COVID-19, visiting patients or staying in the same close environment of a COVID - 19 patients.
- Working together in close proximity or sharing the same classroom environment with a COVID - 19 patient
- Travelling together with COVID-19 patient in any kind of conveyance.
- Living in the same household as a COVID - 19 patients
CASE CLASSIFICATION: [2]
Suspect Case:
A. Patients with severe acute respiratory infection (fever, cough, and requiring admission to hospital), AND with no other etiology that fully explains the clinical presentation AND at least one of the following:
- a history of travel to or residence in the city of Wuhan, Hubei Province, China in the 14 days prior to symptom onset, or
- Patient is a health care worker who has been working in an environment where severe acute respiratory infections of unknown etiology are being cared for.
B. Patients with any acute respiratory illness AND at least one of the following:
- close contact with a confirmed or probable case of 2019-nCoV in the 14 days prior to illness onset, or
- visiting or working in a live animal market in Wuhan, Hubei Province, China in the 14 days prior to symptom onset, or
- worked or attended a health care facility in the 14 days prior to onset of symptoms where patients with hospital-associated 2019-nCov infections have been reported.
Probable case:
A suspect case for whom testing for 2019-nCoV is inconclusive or for whom testing was positive on a pan-coronavirus assay.
Confirmed case
A person with laboratory confirmation of 2019-nCoV infection, irrespective of clinical signs and symptoms
Uncomplicated illness: Patients with uncomplicated upper respiratory viral infection may have nonspecific symptoms such as fever, cough, sore throat, nasal congestion, malaise, headache. The elderly and immunocompromised may present with atypical symptoms. These patients don’t have signs of dehydration, sepsis or shortness of breath.
Mild pneumonia: Patients with mild pneumonia and no signs of severe pneumonia
Severe pneumonia: Fever or suspected respiratory tract infection, plus one of the following:
- Respiratory Rate >30 breaths/min
- Severe respiratory distress
- SpO2 <90% on room air
ARDS: New or worsening respiratory symptoms within one week of known clinical insult. Chest X-ray/ CT/ lung USG: Bilateral opacities not fully explained by effusions, lobar or lung collapse or nodules. Exclude cardiac failure by objective assessment (eg: ECHO).
Mild ARDS: 200 mmHg <PaO2/FiO2 ≤300 mmHg(with PEEP or CPAP ≥5cm H2O, or non-ventilated)
Moderate ARDS: 100 mmHg <PaO2/FiO2 ≤200 mmHg (with PEEP ≥5cm H2O, or non-ventilated)
Severe ARDS: PaO2/FiO2 ≤100 mmHg (with PEEP ≥5cm H2O, or non-ventilated)
Sepsis: Life-threatening organ dysfunction caused by a dysregulated host response to suspected or proven infection, with organ dysfunction. Signs of organ dysfunction include:
- altered mental status
- difficult or fast breathing
- low oxygen saturation
- reduced urine output
- fast heart rate, weak pulse
- cold extremities or low blood pressure
- skin mottling, or
- laboratory evidence of coagulopathy, thrombocytopenia, acidosis, high lactate or hyperbilirubinemia
Septic shock: Persisting hypotension despite volume resuscitation, requiring vasopressors to maintain MAP ≥65 mmHg and serum lactate level < 2 mmol/L.
CURRENT SCENARIO IN INDIA:
The first case of COVID19 in India was reported on 30 January 2020 originating from China. As of 26th March, the Indian Council of Medical Research and Ministry of Family Welfare has confirmed a total of 649 cases (subjected to change in due course), 42 recoveries, 1 migration and 13 deaths in the country. The infection rate of COVID-19 in India is reported to be 1.7, which is remarkably lower than in the worst affected countries. [3]
The outbreak has been declared as an epidemic in more than a dozen states and Union Territories, where provision of the Epidemic Diseases Act, 1897 have been invoked, and educational institutions and many commercial establishments have been shut down. India has suspended all tourist visas, as a majority of cases were linked to other countries. [4]
The Govt. has also issued lockdown of 75 districts across the country where confirmed COVID-19 cases have been reported till 31 March. [5]
Janta Curfew was observed on 22nd March 2020 in the wake of pandemic from 7 am to 9 pm as advised by Prime Minister Narender Modi. [6] He urged all Indians to stay at home for the next few weeks and if possible work from home. [7]
The formation of the COVID-19 Economic Response Task Force was announced during the live address to the nation. [8]
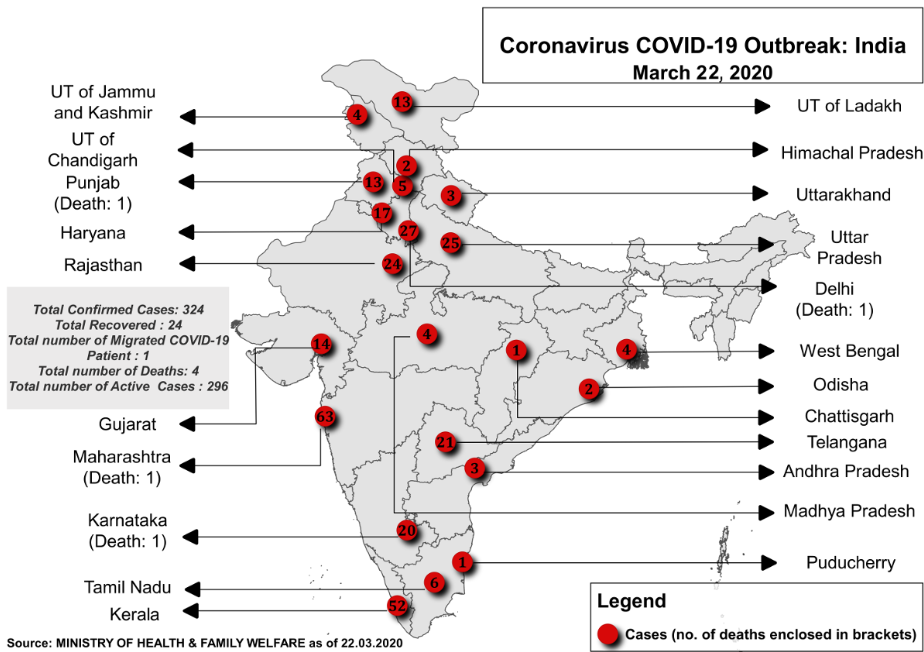

Image 1: Distribution of cases across various states and UTs of India
Source: Ministry of Health and Family Welfare

Common Prevention Measures: [1]
- Clean your hands frequently, either by washing them with soap and water (for at least 20 seconds) or using an alcohol-based (with at least60% alcohol) hand sanitizer (when the hands are not visibly dirty).
- To protect yourself and others, you should always wash your hands: after using the toilet; after handling pets or their waste; before, during and after cooking; before eating and setting the table; after sneezing or coughing and last but not least, wash your hands frequently as possible when you are sick or caring for the sick.
- Cover your mouth and nose with your elbow if you want to sneeze/cough or, preferably, do it in a tissue. Dispose of the tissue immediately in a closed bin.
- Avoid touching your eyes, nose, and mouth with your hands. They have mucous membranes that can act as pathways for particles, and our hands are the primary carrier of those harmful particles.
- Avoid being in direct contact with people that sneeze or cough. Try to maintain a distance of about 6 feet (that is how far the particles can travel) between you and them. Teach your kids to recognize these symptoms and act accordingly. This goes the other way too, stay away from crowded places and avoid contact with people if you are experiencing these symptoms. In other words, maintain social distancing.
- Frequently wipe your phone (especially the screen) with an alcohol based disinfectant. There are many studies that show just how dirty our phones are, with some of them concluding that they are ten times dirtier than a toilet seat. Try to also maintain your children’s phones or smart devices as clean as possible.
- Don’t spit in public. It’s not just rude, but it can spread harmful particles. Explain to your child why he/she should not engage in public spitting. Do it in a tissue that you can safely dispose of.
- If you are sick, stay at home as much as possible. The same goes for a sick family member, encourage them to stay inside and care for them if possible. It decreases the viral load and the risk of spreading, and it’s making your community a safer space for others.
DIAGNOSIS: [2]
Collect blood cultures for bacteria that cause pneumonia and sepsis, ideally before antimicrobial therapy. DO NOT delay antimicrobial therapy to collect blood cultures Collect specimens of nasopharyngeal and oropharyngeal swab for RT - PCR. Clinicians may also collect LRT (Lower Respiratory Tract) samples when these are readily available (for example, in mechanically ventilated patients). Use appropriate PPE for specimen collection (droplet and contact precautions for URT specimens; airborne precautions for LRT specimens). When collecting URT samples, use viral swabs (sterile Dacron or rayon, not cotton) and viral transport media. Do not sample the nostrils or tonsils. In a patient with suspected COVID - 19, especially with pneumonia or severe illness, a single URT sample does not exclude the diagnosis, and additional URT and LRT samples are recommended. Sputum induction should be avoided due to increased risk of increasing aerosol transmission.
In hospitalized patients with confirmed COVID - 19 infection, repeat URT samples should be collected to demonstrate viral clearance. The frequency of specimen collection will depend on local circumstances but should be done at least every 2 to 4 days until there are two consecutive negative results (of URT samples) in a clinically recovered patient at least 24 hours apart.
CURRENT TESTING STRATEGY: [12]
- All asymptomatic people who have undertaken International travel:
- They should stay in home quarantine fo|l4 days.
- They should be tested only ii they become symptomatic (fever, cough difficulty in breathing etc.).
- lf test result is positive, then they should be isolated and treated protocol.
- All contacts of laboratory confirmed positive cases:
- They should stay in home quarantine for 14 days.
- They should be tested only if they become symptomatic (fever, breathing etc.).
- lf test result is positive, then they should be isolated and treated protocol.
- Health care workers managing respiratory distress / Severe Acute Respiratory Illness should be tested if they are symptomatic.
DISCHARGE PROTOCOL: [13]
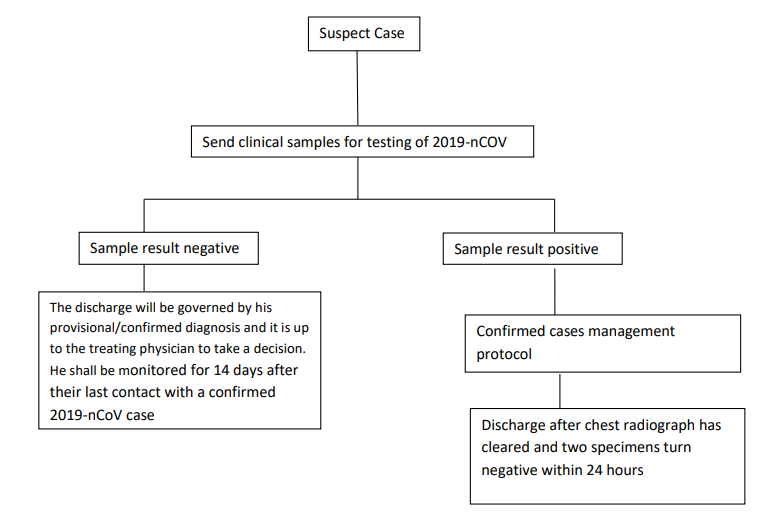
Source: Ministry of Health Family Welfare; Corona Discharge Policy
TREATMENT: [2]
IMPLEMENTATION OF APPROPRIATE IPC (INFECTION PREVENTION AND CONTROL) MEASURES:
At triage:
Give suspect patient a triple layer surgical mask and direct patient to separate area, an isolation room if available. Keep at least 1meter distance between suspected patients and other patients. Instruct all patients to cover nose and mouth during coughing or sneezing with tissue or flexed elbow for others. Perform hand hygiene after contact with respiratory secretions.
Droplet Precautions:
Droplet precautions prevent large droplet transmission of respiratory viruses. Use a triple layer surgical mask if working within 1-2 metres of the patient. Place patients in single rooms, or group together those with the same etiological diagnosis. If an etiological diagnosis is not possible, group patients with similar clinical diagnosis and based on epidemiological risk factors, with a spatial separation. When providing care in close contact with a patient with respiratory symptoms (e.g. coughing or sneezing), use eye protection (face-mask or goggles), because sprays of secretions may occur. Limit patient movement within the institution and ensure that patients wear triple layer surgical masks when outside their rooms.
Contact precautions:
Droplet and contact precautions prevent direct or indirect transmission from contact with contaminated surfaces or equipment (i.e. contact with contaminated oxygen tubing/interfaces). Use Personal Protective Equipment (PPE) (triple layer surgical mask, eye protection, gloves and gown) when entering room and remove PPE when leaving. If possible, use either disposable or dedicated equipment (e.g. stethoscopes, blood pressure cuffs and thermometers). If equipment needs to be shared among patients, clean and disinfect between each patient use. Ensure that health care workers refrain from touching their eyes, nose, and mouth with potentially contaminated gloved or ungloved hands. Avoid contaminating environmental surfaces that are not directly related to patient care (e.g. door handles and light switches). Ensure adequate room ventilation. Avoid movement of patients or transport. Perform hand hygiene.
Airborne Precautions
Ensure that healthcare workers performing aerosol-generating procedures (i.e. open suctioning of respiratory tract, intubation, bronchoscopy, cardiopulmonary resuscitation) use PPE, including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95). Whenever possible, use adequately ventilated single rooms when performing aerosol-generating procedures, meaning negative pressure rooms with minimum of 12 air changes per hour or at least 160 litres/second/patient in facilities with natural ventilation. Avoid the presence of unnecessary individuals in the room. Care for the patient in the same type of room after mechanical ventilation commences.
Management of hypoxemic respiratory failure and ARDS
Recognize severe hypoxemic respiratory failure when a patient with respiratory distress is failing standard oxygen therapy. Patients may continue to have increased work of breathing or hypoxemia even when oxygen is delivered via a face mask with reservoir bag (flow rates of 10-15 L/min, which is typically the minimum flow required to maintain bag inflation; FiO2 0.60-0.95). Hypoxemic respiratory failure in ARDS commonly results from intrapulmonary ventilation-perfusion mismatch or shunt and usually requires mechanical ventilation.
High – flow nasal catheter oxygenation or non – invasive mechanical ventilation: When respiratory distress and/or hypoxemia of the patient cannot be alleviated after receiving standard oxygen therapy, high - flow nasal cannula oxygen therapy or non - invasive ventilation can be considered. If conditions do not improve or even get worse within a short time (1-2 hours), tracheal intubation and invasive mechanical ventilation should be used in a timely manner. Compared to standard oxygen therapy, HFNO reduces the need for intubation. Patients with hypercapnia (exacerbation of obstructive lung disease, cardiogenic pulmonary oedema), hemodynamic instability, multi-organ failure, or abnormal mental status should generally not receive HFNO, although emerging data suggest that HFNO may be safe in patients with mild-moderate and non-worsening hypercapnia25. Patients receiving HFNO should be in a monitored setting and cared for by experienced personnel capable of endotracheal intubation in case the patient acutely deteriorates or does not improve after a short trial (about 1 hr). Implement mechanical ventilation using lower tidal volumes (4-8 ml/kg predicted body weight, PBW) and lower inspiratory pressures (plateau pressure <30 cmH2O). This is a strong recommendation from a clinical guideline for patients with ARDS, and is suggested for patients with sepsis-induced respiratory failure. The initial tidal volume is 6 ml/kg PBW; tidal volume up to 8 ml/kg PBW is allowed if undesirable side effects occur (e.g. dyssynchrony, pH <7.15). Hypercapnia is permitted if meeting the pH goal of 7.30-7.45. Ventilator protocols are available. The use of deep sedation may be required to control respiratory drive and achieve tidal volume targets
In patients with severe ARDS, prone ventilation for >12 hours per day is recommended. Application of prone ventilation is strongly recommended for adult and paediatric patients with severe ARDS but requires sufficient human resources and expertise to be performed safely.
In patients with moderate or severe ARDS, higher PEEP instead of lower PEEP is suggested. PEEP titration requires consideration of benefits (reducing atelectrauma and improving alveolar recruitment) versus risks (end-inspiratory overdistension leading to lung injury and higher pulmonary vascular resistance). Tables are available to guide PEEP titration based on the FiO2 required to maintain SpO2. A related intervention of recruitment manoeuvres (RMs) is delivered as episodic periods of high continuous positive airway pressure [30–40 cm H2O], progressive incremental increases in PEEP with constant driving pressure, or high driving pressure; considerations of benefits vs. risks are similar. Higher PEEP and RMs were both conditionally recommended in a clinical practice guideline. In patients with moderate to severe ARDS (PaO2/FiO2 <150), neuromuscular blockade by continuous infusion should not be routinely used.
In settings with access to expertise in extracorporeal life support (ECLS), consider referral of patients with refractory hypoxemia despite lung protective ventilation. ECLS should only be offered in expert centres with a sufficient case volume to maintain expertise and that can apply the IPC measures required for COVID – 19 patients Avoid disconnecting the patient from the ventilator, which results in loss of PEEP and atelectasis. Use in-line catheters for airway suctioning and clamp endotracheal tube when disconnection is required (for example, transfer to a transport ventilator.) Management of Sepsis and Septic Shock as per hospital protocols.
Role of Antivirals:
There is no current evidence from RCTs to recommend any specific treatment for suspected or confirmed patients with COVID - 19. No specific antivirals are recommended for treatment of COVID – 19 due to lack of adequate evidence from literature.
The use of Lopinavir/ Ritonavir in PEP regimens for HIV (4 weeks) is also associated with significant adverse events which many a times leads to discontinuation of therapy.
In light of the above, Lopinavir/ Ritonavir should ONLY be used with proper informed expressed consent on a case to case basis for severe cases. [7]
Administration of Lopinavir/ Ritonavir to be considered in Laboratory confirmed cases of COVID – 19 when the following criteria are met:
Symptomatic patients with any of the following:
i. hypoxia,
ii. hypotension,
iii. new onset organ dysfunction (one or more)
- Increase in creatinine by 50% from baseline, GFR reduction by >25% from baseline or urine output of <0.5 ml/kg for 6 hours.
- Reduction of GCS by 2 or more
- Any other organ dysfunction
iv. High Risk Groups:
- Age> 60 yrs
- Diabetes Mellitus, Renal Failure, Chronic Lung disease
- Immunocompromised persons
Dosage:
Lopinavir/ Ritonavir (200 mg/ 50 mg) – 2 tablets twice daily. For patients unable to take medications by mouth: Lopinavir 400mg/ Ritonavir 100 mg – 5ml suspension twice daily for 14 days or for 7 days after becoming asymptomatic.
POSSIBLE ROLE OF REMDESIVIR:
Remdesivir is a novel antiviral drug in the class of nucleotide analogs. It was developed for use against Ebola virus disease and Marburg viral infections. [14]
Mechanism of Action: It is a prodrug that interferes with action of viral polymerases and evades proofreading by viral exoribonuclease, causing a decrease in viral RNA production
Remdesivir was developed to treat several other severe viral diseases, including the disease caused by Ebola virus. Also in late January 2020, remdesivir was administered to the first U.S. patient confirmed to be infected by SARS-CoV-2 after he progressed to pneumonia.
While no broad conclusions can be made based on the single treatment, the patient's condition improved dramatically the next day, [15] and he was eventually discharged. [16]
WHO had announced the launch of a large four-arm pragmatic clinical trial (SOLIDARITY trial) that includes one group of patients treated with remdesivir. [17]
Two large randomized clinical trials are underway in China. The two trials will enroll over 700 patients, and are likely to definitively answer the question of whether the drug is effective in treating COVID-19.
The results of those studies are expected in April or May 2020. Studies also are underway in the United States, including at several Harvard-affiliated hospitals.
It is hard to predict when the drug could be approved for use and produced in large amounts, assuming the clinical trials indicate that it is effective and safe. [18]
PROPHYLAXIS: [19]
The National Task Force for COVID-19 constituted by Indian Council of Medical Research recommends the use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection for high risk population. The Advisory provides for placing the following high risk population under chemoprophylaxis with hydroxychloroquine;
- Asymptomatic healthcare workers involved in the care of suspected or confirmed cases of COVID-19.
- Asymptomatic household contacts of laboratory confirmed cases.
Possible Mechanism: It is a weak base known to elevate the pH of intracellular organelles, essential for membrane fusion. [20] HCQ also inhibited the entry step as well as the post entry stages of SARS- CoV-2 infection Since acidification is crucial for endosome maturation and function, it was summarised that endosome maturation might be blocked at intermediate stages of endocytosis, resulting in failure of further transport of virions to the ultimate releasing site. Clinical investigation found that high concentrations of cytokines were detected in the plasma of critically ill patients infected with SARS-CoV-2, suggesting that cytokine storm was associated with disease severity. [21] Other than its direct antiviral activity, HCQ is a safe and successful anti-inflammatory agent that has been used extensively in autoimmune diseases and can significantly decrease the production of cytokines and, in particular, pro-inflammatory factors. Therefore, in COVID-19 patients, HCQ may also contribute to attenuating the inflammatory response. [22]
WHAT NEEDS TO BE DONE?
Government and doctors and paramedics are working to the best of their services. People should follow and adhere to Govt. advisories strictly. Indians should take lessons from China and Italy that what havoc this novel virus can create. The need of the hour is social distancing and as I am writing this article Govt has already put many districts under lock down keeping in view the rise in the number of cases and keeping the condition in the phase 2 of the outbreak. The citizens should understand their responsibility positively that it is not for others or for Govt., it is for their selves and for their families. What goes the future of COVID-19 in India is not known but one thing is for sure if we follow social distancing protocol and advisories strictly, we can make way for lives of ours and our dear ones and can prevent the coming cyclone hovering over India right now.
STAY SAFE. STAY AT HOME
REFERENCES
- Bradley M. The Essential Guide to The Wuhan Virus (Symptoms, Transmission and Prevention). Corona Virus ; 2020
- Guidelines on Clinical Management of COVID-19. Available from: https://www.mohfw.gov.in/pdf/GuidelinesonClinicalManagementofCOVID1912020.pd
- Sinha A. One COVID-19 positive infects 1.7 in India, lower than in hot zones.The Indian Express; 23 March 2020.Available from: https://indianexpress.com/article/coronavirus/coronavirus-india-infection-rate-china-6321154/
- Sanyal A."India Suspends All Tourist Visas Till April 15 Over Coronavirus: 10 Facts" . NDTV.com. Retrieved 12 March2020. Available from: https://www.ndtv.com/india-news/coronavirus-impact-visas-to-india-suspended-till-april-15-2193382
- Hebbar, Nistula. Coronoavirus: Union government announces lockdown in 75 districts till March 31.The Hindu; 22 March 2020.Available from:https://www.thehindu.com/news/national/coronavirus-india-locks-down-80-districts-to-contain-virus-spread/article31134567.ece
- Bureau, Our. "PM Modi calls for 'Janata curfew' on March 22 from 7 AM-9 PM". Businessline;19 March 2020.
- Modi appeals for self-imposed janata curfew on Sunday". Business standard; 19 March 2020.
- Covid 19 Economic Task Force: Government forms Covid-19 economic response task force, says PM Modi". The Times of India; 20 March 2020
- Coronavirus outbreak in India.Available from: https://commons.wikimedia.org/wiki/File:COVID-19_Outbreak_Cases_in_India.svg
- Who needs Mask. Available from: https://www.mohfw.gov.in/pdf/Mask-Eng.pdf
- Do’s and Dont’s in Covid19.Available from: https://www.mohfw.gov.in/awareness.html
- Revised Strategy of Covid-19 testing in India. Available from: https://www.mohfw.gov.in/pdf/ICMRrevisedtestingstrategyforCOVID.pdf
- Discharge policy of nCov Cases. Available from: https://www.mohfw.gov.in/pdf/Corona%20Discharge-Policy.pdf
- Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys". Nature 2016; 531 (7594): 381–5
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. (Washington State 2019-nCoV Case Investigation Team)."First Case of 2019 Novel Coronavirus in the United States". The N Eng J of Med 2020; 382(10) : 929-36
- Harmon A. "Inside the Race to Contain America's First Coronavirus Case". The New York Times Feb 2020
- "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". UN News. March 2020
- Ellerin T, Farid H, Komaroff A, Krakower D, Lewine HE, Marques L, et al. As coronavirus spreads, many questions and answers. Coronavirus Resource Centre. Harvard Health Publishing. Available from: https://www.health.harvard.edu/diseases-and-conditions/coronavirus-resource-center
- Advisory on the use of hydroxychloroquine for the prophylaxis of SARS-nCoV infection. Available from: https://www.mohfw.gov.in/pdf/AdvisoryontheuseofHydroxychloroquinasprophylaxisforSARSCoV2infection.pdf
- Mauthe, M. et al. Chloroquine inhibits autophagic flux by decreasing autophagosome -lysosome fusion. Autophagy 2018 ;14:1435–55.
- Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 ; 395:497–506
- Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H et al. Hydroxychloroquine, a less toxic derivative of chloroquine is effective in SARS-CoV2 infection in vitro. Cell Discov 2020; 6(16).
How to cite this article: Kachroo V. Novel coronavirus (COVID-19) in India: current scenario. International Journal of Research and Review. 2020; 7(3): 435-447.
******


